
Social Evolution of Bees Through Environmental Pressure
By McKenna Kilburg '18
BIOL-320: Evolution
The assignment consists in writing a research proposal that investigates an evolutionary question. Students have freedom to choose their specific topic. I thought McKenna’s proposal was especially interesting because it focused on the evolution of social behavior by investigating a species that shows polymorphism for the traits.
–Paulina Mena
Introduction
Over eight million different species exist on earth, and of these, most species display some sort of socialization within or between species. This socialization can take the form of several frameworks. These include team reciprocity, in which one organism will trust another until they are crossed, or selfish teamwork, where members of a group act together to achieve a common goal that helps all parties individually. In addition, many species utilize altruism, in which all actions taken are for the good of the group rather than a specific individual (Dugatkin, 1999). The most organized form of altruism, and sociality in general, is termed eusociality, which is characterized by overlapping generations, cooperative care for the young, and differential reproductive castes (Andersson, 1984).
One taxa that consistently displays eusociality is the order of Hymenoptera, which consists of wasps, bees, and ants. This group of organisms is unique because of their sex-determination process termed haplodiploidy. Like all Hymenoptera, when a female bee mates, she can store the male’s sperm in a specialized organ called a spermatheca. This sperm can be utilized to fertilize an egg, creating a diploid female. Conversely, an unfertilized, haploid egg develops into a male. (Zayed and Packer, 2009).
Bee species lie on a spectrum of sociality, with some being completing solitary in their singular nests, others being primitively social, in which they maintain brood care or share a nest, and others being eusocial in which they share a nest in addition to the tasks needed to maintain their community. This sex differentiation helps to form role distinctions within a colony. A haploid male’s only role is to simply feed until the queen is ready to mate; in some species, they are also responsible for guarding the nest. Conversely, females either take on the role of the queen or a worker depending on their allotted nutrients as larvae. Workers are fed pollen and nectar, whereas an upcoming queen is fed royal jelly, but this only occurs in highly social species (Wilson-Rich, 2014).
This differentiation between a female queen and worker fulfills one of the eusociality requirements in that it creates differential reproductive castes. The queen bee mates with select drones and lays thousands of eggs at a time, which the workers then care for. However, the worker bees are incapable of reproducing due to the difference in their nutrients that turns certain genes off and on; in addition, many queens release pheromones that inhibit a worker bee’s ability to reproduce (Wilson-Rich, 2014). This appears to be troublesome for the theory of evolution because an individual’s fitness is determined by its ability to survive and reproduce (Bergstrom and Dugatkin, 2012). However, most of the bees within a colony are not capable of reproducing and would be deemed evolutionary impasses, yet bees still seem to be evolutionarily favored as sterile females continue to survive. This paradox has puzzled scientist for generations. In fact, Charles Darwin, himself, claimed that the sterile female workers represent a “special difficulty, which at first appeared to me insuperable, and actually fatal to my whole theory” (Darwin, 1871).
Despite the initial confusion, Darwin proposed that sterile individuals can be evolutionarily fit if they pass on their own genetic information by helping the queen reproduce. This is possible because bees, and all haplodiploid species, are highly related. Sister bees share three-quarters of their DNA with one another, but a bee only shares 50% of their DNA with their own offspring, as shown in Figure 1, below. As a result, it is more beneficial to help individuals that are more related to oneself survive rather than reproducing on one’s own. Therefore, it has been proposed that relatedness is the driving force that allows eusociality to evolve.
This idea is referred to as kinship selection, and it was first proposed by William Hamilton, an evolutionary biologist, in 1964 (Queller and Strassmann, 1998). This theory explains that organisms are likely to behave altruistically toward their own family members in order to help perpetuate their genetic lineage. Kinship selection theory has grown synonymous with haplodiploidy because the unparalleled amount of relatedness in haplodiploids makes them the most likely to show kinship favoritism (Nowak et al., 2012). Therefore, worker bees are willing to sacrifice their own lives, by foraging for food or guarding a nest, because this effort helps to propagate their genetic lineage. This theory of kinship selection also explains that worker bees tend to the queen’s offspring because workers can perpetuate their genetics by caring for the next generation of their family. In Hamilton’s original theory, he measured the worth of an action based on the equation rB > C, where the relatedness of an individual (r) multiplied by the benefits received by the action’s recipient (B) must outweigh the cost absorbed by the individual performing the action (C) for said action to be worth acting on (Queller and Strassmann, 1998).
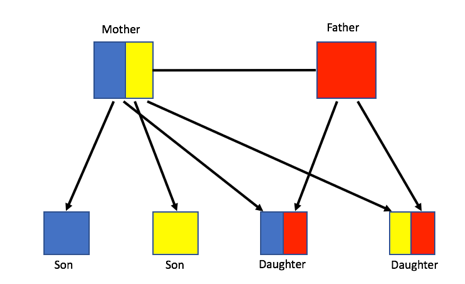
Figure 1: Haplodiploid relatedness showing that sisters are more related to one another than their own offspring.
Kinship selection has been used as the textbook explanation as to how eusociality originates for decades. However, Nowak et al. (2012) show that an increasing number of exceptions continue to surface as several species that are diplodiploid show sociality, such as species of shrimp and mole rats, in addition to many haplodiploids not displaying sociality, like the many solitary bee species. As a result, other theories have been proposed to determine the origin of sociality.
Mutualism was offered as an explanation for eusociality as groups of bees that live together all benefit from the shared home that offers protection from predators. In addition, each bee has a specific task of nursing larvae, tending to the queen, guarding the hive, or foraging, and each individual bee benefits from the work of all the other bees, thus making social interaction evolutionarily favorable (Wilson-Rich, 2014; Andersson, 1984). However, mutualism does not explain why sterile workers are necessary; this caveat lead to the theory of manipulation, in which worker bees are coerced into servicing the colony because the most fertile female bees release reproduction-inhibiting chemicals. In addition, any worker that manages to produce offspring despite the inhibiting pheromones may be eaten by the queen to discourage such autonomy (Ratnieks and Wenseleers, 2008). Although manipulation explains the caste system and cooperative brood care, it does not explain why sterile workers choose to stay within the social hive rather than beginning their own solitary nest (Andersson, 1984).
Over all, no theory explaining the origin of eusociality has been entirely accepted by the scientific community. Nonetheless, it has been noted that there are several traits in common between all eusocial species. Therefore, it has been proposed that some pre-conditions are required to later evolve eusociality. The two most basic traits include a cohesive nest and a delivery system to get nutrients to the nest. Dr. Malte Andersson, a professor of Animal Ecology at the University of Gothenburg in Sweden, hypothesized that the presence of these traits allowed for opportunities of social engagement and task delegation; without the help of other bees, constructing a hive and finding food is a sizable chore, so working with others creates a more efficient use of energy (Andersson, 1984). Additionally, Dr. Sarah Kocher and Dr. Robert Paxton, from Harvard University and Martin-Luther University, respectively, found that a lesser form of sociality, called communality, where females share a nest together but do not have reproductive castes, is a pre-adaptation to eusociality (Kocher and Paxton, 2014). Despite these findings, several solitary species also exhibit these pre-eusocial traits, indicating that they will either evolve sociality in the future, or more comprehensive traits are needed to diagnose social species.
With this knowledge, several studies have attempted to investigate the causal agent of sociality within previously solitary bees. One study, conducted by several professors and students at Cornell University, evaluated molecular changes that could serve as pre-adaptations to eusociality. By sequencing and comparing the DNA of bee species, they quantified the amount of variation between advanced eusocial, primitive eusocial, and non-eusocial bees. It was found that genes associated with gland development, signal transduction, carbohydrate digestion, and brain development evolve more quickly in the eusocial lineages, suggesting that these molecular changes could be the pre-adaptions, or the “genetic toolkit,” for the evolution of eusocial lineages (Woodard et al., 2011).
Additionally, other studies have looked at environmental factors that could serve as selective pressures for the evolution of eusociality. One in particular, conducted by Norman Lin, a professor of Zoology at the University of Kansas, showed a positive correlation between the presence of predators and parasites and social behaviors in bees. His study demonstrated that an increased amount of dangers in the local environment causes insects to congregate into one successful location; if an abundance of insects make up this community, then some members can serve as guards for the rest, creating the first of the divided hive tasks (Lin, 1964).
In addition, two German professors, Dr. Ingolf Steffan-Dewenter and Dr. Susanne Schiele from the University of Bayreuth and University of Gottingen, respectively, instead evaluated whether the population dynamics of native bees were regulated by bottom-up and top-down forces. Over the course of five years, thirty populations of solitary bees were monitored in fragmented habitats, and it was determined that available foraging area had a larger effect on population size than predators. Therefore, they concluded that bottom-up forces, such as nesting resources and food availability, determine the success of a population’s size and social tendencies. (Dewenter & Schiele, 2008)
Statement of Problem
The evolutionary method of developing sociality remains unknown because no one proposed process has been entirely accepted by the scientific community. Many studies have shown that genetic and environmental factors can serve as pre-adaptations to sociality in insects, including DNA mutations or the presence of predators and parasites. However, no study has completely isolated one bottom-up environmental force that can lead to sociality. Therefore, this study hopes to distinguish such a force and show that it prompts social behaviors in bee populations.
Significance of Project
It is necessary to put research efforts toward investigating the effects of bottom-up forces because humans have a direct impact in limiting suitable habitats and resources for insects through deforestation and habitat fragmentation. This proposed research intentionally deprives bees of these nesting resources to determine their ability to adapt. The goal of this study is to obtain information that can be used to further conserve native populations of bees.
If this isolated study can show a correlation between nest resource depletion and increased social behaviors, then it can be concluded that limited resources in the environment can lead to more social organization within insect populations. Therefore, the presence of nesting resources acts as a selective pressure in a population in which only the adaptably social can survive, leading to increased evolutionary fitness in those that are social in these situations. Contrarily, if this study shows that limited nesting resources cause increased mortality in bees, then it can be concluded that deforestation and habitat fragmentation are harmful to local bee populations because they cannot adapt to areas without nesting resources.
Hypothesis
In this specific study, the effects of depriving Ceratina bees, also known as small carpenter bees, a genus with social pre-adaptations, from nesting resources will be studied. It is hypothesized that there will be a significant difference in the preference between singular or shared nesting and a significant difference in the growth and survival rates of the offspring from singular and shared nesting. Without an excess of nesting resources, bees with social pre-adaptions should be able to adapt to sharing resources and forming colonies than bee populations without pre-conditions. This prediction is based on evidence from other studies showing that social pre-adaptations are a beginning step toward future sociality (Kocher & Paxton, 2014; Andersson, 1984).
Methods
In this proposed study, the Ceratina bee genus will be studied because of its polymorphic social behavior. These small carpenter bees create their nests within the shoots of plants. Each spring, Ceratina bees emerge from overwintering and begin preparing for the mating season ahead. The females find a suitable home and begin to excavate a nest into it, creating an entrance hole and a long tunnel about 4-8 inches in length (Rehan & Richards, 2010). Some Ceratina bees will find solace in old nests used in past seasons. The foundress who creates or finds this nest will then find a male mate, become fertilized, and begin collecting pollen and nectar that will feed her offspring. She rolls up her pollen collection and positions it at the end of her tunnel where she lays a single egg on top of the pollen mass. She then builds a wall with chewed wood pieces, closing off the brood cell.

Figure 2: Ceratina bee larvae housed in individual brood cells within a plant shoot, scale in mm (Ali et al 2016).
The foundress continues this process until there is no room for another brood cell; an example of this is depicted in Figure 2. The Ceratina eggs hatch within a matter of days and the resulting larvae will feed on the provided pollen mass; once sufficiently fed, the larvae pupate within the cell, and emerge as an adult after nearly two months by excavating out of the shoot.

Catalina Valdez, linoleum print, 24” x 24”
Most often, Ceratina bees are sub-social, which means that they show extended parental care for their offspring. However, one species, Ceratina calcarata, has an additional social pre-adaptation in which they create a small reproductive caste system where one daughter is coerced into caring for her sisters and forgoing her own reproduction. The first brood cell created by the foundress is given significantly less pollen that is more rich in proteins; this daughter emerges earlier than her sisters and is also much smaller. This dwarf eldest daughter serves her family most likely because of altruistic kinship in that she can only pass on her genetic information by helping her sisters survive to procreate (Lawson et al., 2017). Despite C. calcarata’s additional social pre-adaptation, there has not been a more substantial division of labor within the Ceratina genus, such as that of the eusocial queen, workers, and drones, nor the presence of multiple generations living in one nest.
Previous studies have shown that progression from a solitary status, where there is no brood care, to one of sub-sociality is a preadaptation toward later eusociality (Kocher & Paxton, 2014; Andersson, 1984). In addition, specific Ceratina species have developed even further social adaptations like the small separation of labor between sisters. Therefore, it can be hypothesized that Ceratina bees are likely to develop increased social behaviors if given the correct environmental pressures given their social pre-adaptations.
In this study, Ceratina bees will be captured at Central College’s Field Station within their overwintering nests. These small carpenter bees hibernate within old nests and emerge in late spring. The males emerge from these nests in early May, and the females typically emerge three weeks later (Rehan & Richards, 2010). Therefore, several overwintering nests will be obtained in March, most likely found within sumac and raspberry plants. These nests will be identified by the single, circular entrance hole, about one-half centimeter in diameter, where a female carpenter bee had initially excavated the nest (Lawson et al., 2016; Ali et al., 2016). A minimum of five occupied nests of varying Ceratina species will be collected by carefully covering the entrance hole with tape so no occupants escape in addition to using a hand saw to break off the nest from the identified branches (Rehan & Richards, 2010). These still-overwintering nests will be gently placed into one enclosed greenhouse. Here, the bees will continue to hibernate until May. Once emerged, the old nests will be removed and the occupants will search for food, shelter, and mates.
However, the greenhouse’s resources will be limited. There will be an abundance of angiosperms for the bees to collect nectar and pollen from, namely annuals like geranium, petunias, and lilies because these plants contain stems in which bees cannot excavate. In addition, nesting will be provided for the bees. The greenhouse will contain a limited number of dead, broken twigs from raspberry or sumac plants for the Ceratina bees to excavate. The number of available twigs will depend on the number of Ceratina bees that successfully emerge from overwintering. These twigs will have a glass plate covering one side, allowing researchers to see the bees’ behaviors inside their created nest. However, there will not be enough of these twigs provided for each female bee to continue their sub-social lifestyle of one foundress per shoot. There will also be no available wood in the greenhouse for the carpenter bees to use as additional nesting materials.
With the bees’ resources limited, their behavioral tendencies like nest claiming, food foraging, and interacting with others are likely to be altered. Typically, each female foundress will forage for pollen and find a nest on her own, but with limited resources, the Ceratina bees will either fight over the only nesting options available, share the nesting area, or die from failure to find a nest. Because the Ceratina bee genus has various pre-adaptations to sociality with their parental care for their offspring and minor reproductive castes, it is likely for them to adopt the social behavior of nest sharing and potentially sharing brood care responsibilities.
Observations of the bees’ behaviors will be recorded twice each day, making sure to note the number of bees within one nest and the number without nests in addition to the amount of help non-mothers contribute to brood care, the number of eggs deposited, and the survival of the offspring within the nests. These observations will take place over the course of four months, from May to September so the original overwintering population’s offspring will be seen emerging from their brood cells. The bees that inhabit a nest solitarily or do not find a nest will be compared to those that co-inhabited nests. A student’s t-test will be used to compare the number of bees in single or shared nests in addition to comparing the growth and survival rates of the offspring from the single and shared nests. This will determine if there is a significant difference in the social preference of Ceratina bees if resources are limited and if one social structure is more beneficial for the offspring.
If the Ceratina bees show a significant preference for sub-social nesting, the researcher can conclude that another environmental factor, outside of resource depletion, causes the drive toward sociality, such as food depletion or the presence of predators. However, if the Ceratina bees significantly adopt social tendencies, then the researcher can conclude that limited nesting resources is a driving environmental pressure toward sociality. Therefore, if nest resource depletion continues in the environment, it can be expected that the Ceratina bee lineage will adapt to this selective pressure, allowing those who can share nests to survive and reproduce. Over time, Ceratina bees may become accustomed to sharing nests and develop a higher level of social structure.
Follow-Up Study
A later study will be conducted in which food availability is the independent variable rather that nesting resources. In this case, the enclosed greenhouse would have an abundance of nesting sites available, but there would only be a limited number of food sources. For example, only one petunia plant could be placed in the greenhouse. The bees’ behavior to this environmental pressure would be evaluated in the same manner as previously mentioned, but simply in relation to a different variable. Therefore, if this second experiment were to create a significant difference in the social preference of Ceratina bees, researchers could conclude that food resource depletion is a driving environmental pressure toward sociality. However, it is important to conduct these two experiments completely separately as to confidently determine which factor relates more to social evolution. It could be possible that both limited nesting and food resources cause social tendencies in bees, but this ought to be determined separately.
Works Cited
Andersson M. 1984. The evolution of eusociality. Ann. Rev. Ecol. Syst. 15: 165-189.
Ali H, Alqarni A, Shebl M, Engel M. 2016. Notes on the nesting biology of the small carpenter bee Ceratina smaragdula (Hymenoptera: Apidae) in Northwestern Pakistan. Florida Entomologist. 99(1): 89-93.
Bergstrom C, Dugatkin L. 2012. Evolution. New York (NY): W.W. Norton & Company.
Darwin C. 1871. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. New York (NY): D. Appleton & Company.
Dewenter I, Schiele S. 2008. Do resources or natural enemies drive bee population dynamics in fragmented habitats. Ecology. 89(5):1375-1387.
Dugatkin, L. 1999. Cheating monkeys and citizen bees. New York (NY): The Free Press.
Kocher S, Paxton R. 2014. Comparative methods offer powerful insight into social evolution of bees. Apidologie. 45: pp. 289-305.
Lawson S, Ciaccio K, Rehan S. 2016. Maternal manipulation of pollen provisions affects worker production in a small carpenter bee. Behav Ecol Sociobiol. 70(11): 1891- 1900.
Lawson S, Helmreich S, Rehan S. 2017. Effects of nutritional deprivation on development and behavior in the subsocial bee Ceratina calcarata (Hymenoptera: Xylocopinae). Journal of Experimental Biology. 22: 4456-4462.
Lin N. 1964. Increased parasitic pressure as a major factor in the evolution of social behavior in halictine bees. Insectes Sociaux. 11: 187-192.
Nowak M, Tarnita C, Wilson E. 2010. The evolution of eusociality. Nature. 466: 1057-1062.
Queller D, Strassmann J. 1998. Kin selection and social insects. BioScience. 48(3): 165-175.
Ratnieks F, Wenseleers T. 2008. Altruism in insect societies and beyond: voluntary or enforced? Trends Ecol. Evol. 23: 45-52.
Rehan S & Richards M. 2010. Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Entomological Society of Canada. 142(1): 65-74.
Wilson-Rich N. 2014. The Bee: a natural history. Princeton (NJ): Princeton University Press.
Woodard S, Fischman B, Hudson M, Varala, K, Cameron S, Clark A, Robinson G. 2011. Genes involved in convergent evolution of eusociality in bees. PNAS. 108(18): 7472-7477.
Zayed A, Packer L. 2007. The population genetics of a solitary oligolectic sweat bee, Lasioglossum (Sphecodogastra) oenotherae (Hymenoptera: Halictidae). Heredity. 99: 397–405.